 Tamayakin’ initiative
Tamayakin’ initiative
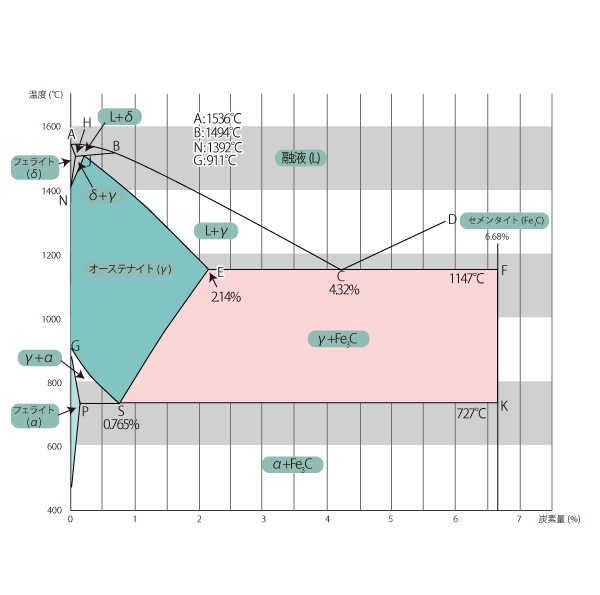
Iron-Carbon Equilibrium Diagram

 Return to list
Return to list
Overview.
Steel is an alloy of iron and carbon. The iron-carbon equilibrium diagram is an equilibrium state diagram with temperature on the vertical axis and carbon content on the horizontal axis.
01 Iron-Carbon Equilibrium Diagram
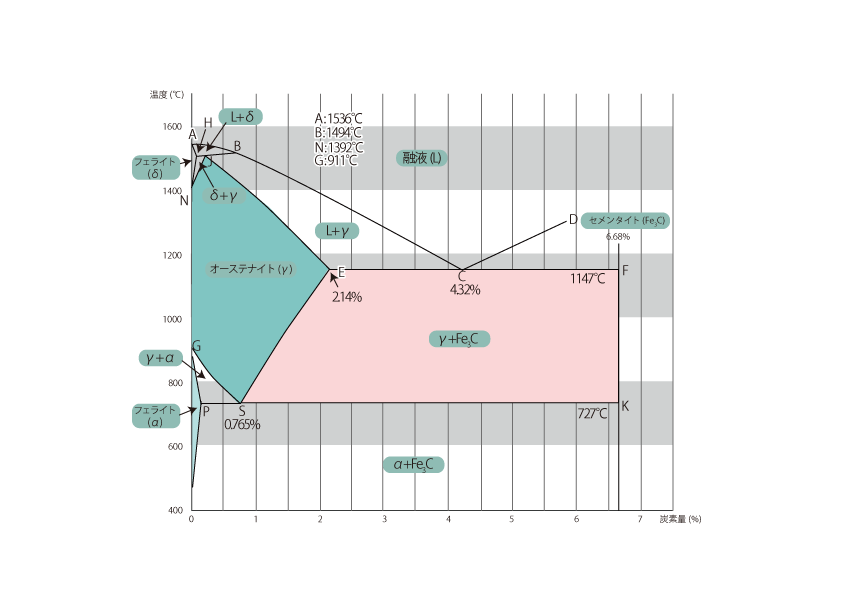
Steel is an alloy of iron and carbon. The equilibrium state diagram of the iron-carbon system is shown above as an equilibrium state diagram of the amount of carbon and at various temperatures. However, this equilibrium state diagram is for the case where carbon is cementite, and is for the case of practical steel.
ABCD in the figure represents Liquid phase lines The temperatures above these temperatures are liquid. Above these temperatures, the liquid phase becomes liquid.
Other solid lines are transformation lines and include the A1, A3, and Acm lines. In the transformation lines, the response to gradual cooling is shown below.
(1) Line A1 (solid PSK ): The temperature at which the transformation from austenite to ferrite + cementite ( Fe3C ) begins, which is constant at 727°C in equilibrium, regardless of carbon content.
(2) Line A3 (solid line GS): This is the temperature at which the transformation from austenite to ferrite begins. The higher the carbon content, the lower it becomes, and it coincides with the A1 line at 0.765%. This transformation line is exists only in sub-eutectoid steel. and is the criterion for determining the complete annealing, annealing, and quenching temperatures for sub-eutectoid steel.
(3) Line A4 (solid line HN): Temperature at which transformation from δ-ferrite to austenite begins. the N point is 1392°C, so this transformation line is irrelevant for heat treatment of steel.
(4) Acm line (solid line SE): This is a transformation that exists only in hypercovalent steel and is the temperature at which cementite ( Fe3C ) begins to precipitate from austenite. The Fe3C precipitated when passing through this Acm transformation point is called primary Fe3C.
(5) Other transformation points: Although no microstructural change occurs, there is a magnetic transformation of pure iron at 770°C and a magnetic transformation of cementite at 210°C. At these temperatures, the material changes from paramagnetic to ferromagnetic.



